Manufacturing and Formulation Impacts on Colloidal Speciation in ASD Dosage Forms
View Now!
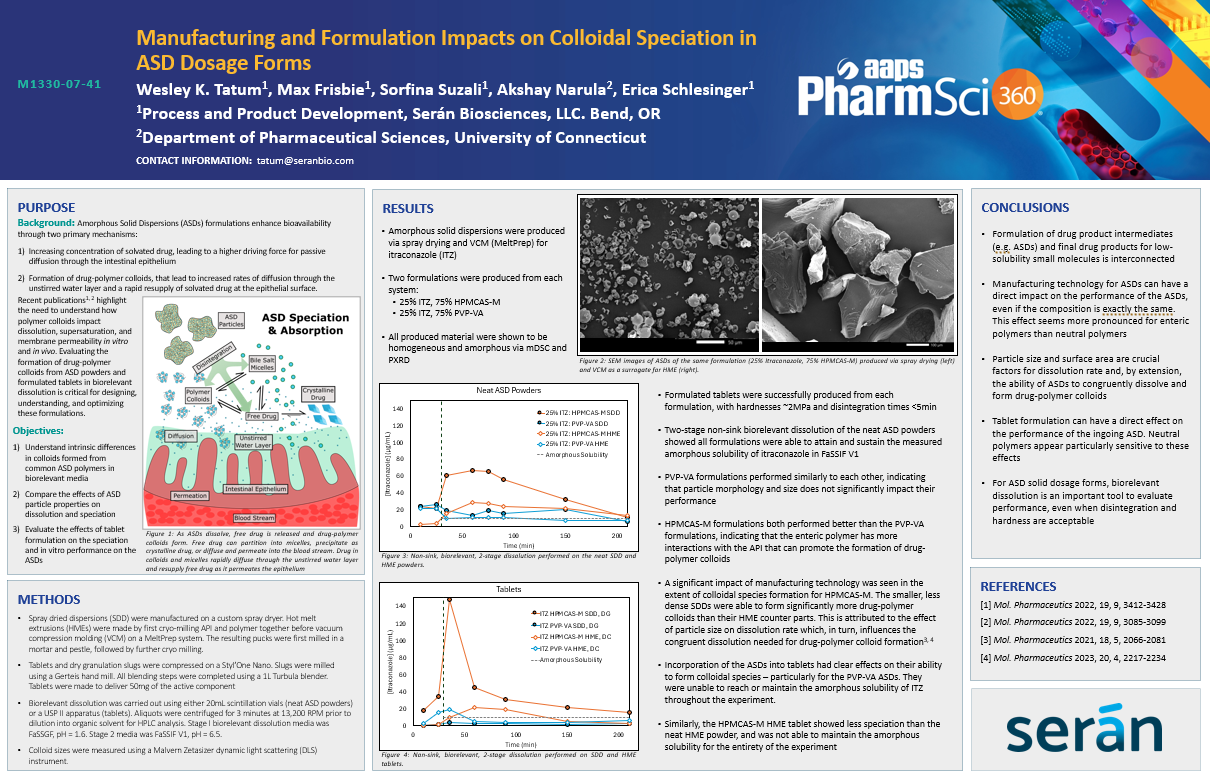
Amorphous solid dispersions (ASDs) have emerged over the past decade as the leading formulation technology for low solubility compounds.
In this work, we characterized the colloids formed by polymers traditionally used in ASDs and identified intrinsic differences in size and density as a result of chemistry, hydrophobicity, and polymer concentration in biorelevant media. Building off of these results, we evaluated the degree to which model drug compounds spontaneously partition into polymer colloids and measured their effect on the nature of colloidal species. The 4 model compounds were selected to have a range of physicochemical properties.
Key Learnings:
- It is critical to consider the effects of formulation and processing as early as possible.
- The ability of the ASD to promote colloidal speciation can be enhanced or diminished by the many aspects of formulation.
- Knowledge of the intrinsic polymer properties, intermolecular interactions with the API, and dependence on dissolution rate are critical to creating robust, advanceable, and scalable oral dosage forms.
View Poster
About the Author

Wesley Tatum, PhD
Principal Engineer
Process & Product Development
Wesley Tatum is a Principal Engineer in the Process and Product Development department at Serán. He leads a team of scientists and engineers in characterizing new API and identifying shortcomings for bioavailability. He and his team then screen and select drug product intermediates that address those shortcomings and screen and scale up drug product formulations. During his time at Serán, his work and research has focused on understanding how polymers, excipients, and physiological factors influence speciation, bioavailability, and absorption of drug substances and amorphous solid dispersions.
Wesley holds undergraduate degrees in Physics and Physical Chemistry from Whitworth University and a Ph.D. in Materials Science and Engineering from the University of Washington.
About Serán
There's a science to success.®
Serán is a leading science-based CDMO that specializes in a variety of drug delivery and formulation approaches suited to optimizing bioavailability. Serán provides capsules, tablets, multi-particulates, and powder-in-bottle formulations. Our solid dosage forms are engineered for a wide range of formulation approaches such as overcoming solubility challenges and to enable extended-release.
-1.png?width=250&height=80&name=Ser%C3%A1n%20Logo%20(Blue%2c%20Transparent%20Background)-1.png)